Challenge
Industry 4.0 requires better data access. Therefore, the company needed to replace their closed non-expandable honeywell GR series graphic chart record. Their old system recorded the data in a proprietary data structure, which needed a separate product to access. That meant they had less security and non-flexible reporting.
Solution
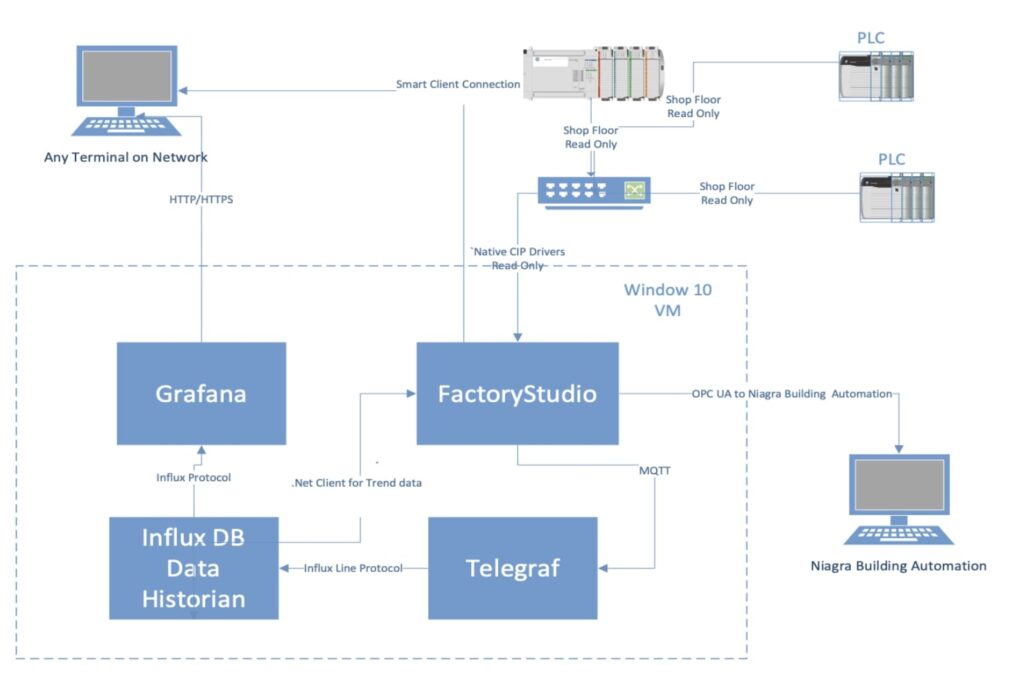
The company chose FactoryStudio powered by FrameworX because it provided a full Solution for Trending, Alarming, Reporting, data storage and graphic displays with redundancy and multiple remote clients. FactoryStudio also includes many real-time devices drivers like OPC UA and an MQTT Broker to allow secure access to other systems.
Results
With the new system, the client could use data historian technology redundancy to handle the increasing data loads and storage requirements while maintaining acceptable user response. They also gained:
- Remote access to the data and open access to recorded data, making reporting easier and faster.
- Enhanced trending functions, replacing paper chart recorders and increasing security.
- Flexible security to meet FDA 21 CFR Part 11
“FrameworX was the perfect SCADA platform to meet our sterile Environment Monitoring system requirements. Its built-in features allow for full CFR Part 11 compliance out of the box. From the low-cost remote clients, integrated data historian, redundancy, and ease of customization, are key value adds, with no hardware vendor lock-in.”
— Sam Khodak, Pharmaceutical Data Integrity Specialist
The FDA requires a validated system for monitoring, alarming, trending, and reporting of environmental sensors in sterile environments. These systems are also subject to FDA 21 CFR Part 11 requirements, which include audit trails, password rotation and strength policies, and electronic record verification with e-signatures.
Traditionally, pharmaceutical companies have used closed stand-alone or vendor-specific systems to handle these requirements. However, there is an increasing need for this information to be available to operations, quality analysts, and planners for production decisions (i.e., batch quality assessment, cleaning requirements, capacity planning).
There is also a need for systems that are flexible enough to meet FDA regulations and security requirements and are open enough to distribute key production data to the users who need it.
FrameworX was chosen for system requirements based on the following factors:
- Built-in historian (Tatsoft Canary Historian from Canary Labs)
- FDA 21 CFR Part 11 features (LDAP, e-signature, custom audit trails)
- Open environment and .NET scripting language
- Enhanced trending functions
- Client-server model allowing economical access for unlimited remote users
- Redundancy included in the base system
- Works with multiple data sources for trending and alarms
- Pricing – low base system cost, low remote client cost, free 500-tag historian
- No vendor lock-in – works with nearly all major PLCs and sensor vendors via native drivers
Benefits:
- Replacement physical chart recorders
- Electronic records with database security and CFR Part 11 compliance
- Built-in reporting with PDF and XPS formats
- Open access to recorded data via SQL and data historian APIs
- Open access to real-time values via OPC UA and MQTT
- Enhanced trending functions with custom calculations and statistics (i.e., MKT)
- Native drivers to read data from PLCs and instrumentation via numerous protocols
Controlware: our Tatsoft System Integrator Partner
Controlware, LLC has been providing automation system design, development, and integration services for manufacturing organizations since 1990. They deliver solutions ranging from SCADA, Manufacturing Execution Systems, and batch management in regulated industries. They also specialize in integration with Enterprise Resource Planning, Supply Chain Management, and existing business systems.
To be competitive in today’s global marketplace, manufacturing excellence is not enough. Modern businesses require the ability to quickly analyze data from Finance, Sales, Marketing, and Engineering as well as the shop floor, and convert it into information that supports management with effective decision-making. This requires the ability to communicate the results of those decisions effectively with clients, employees, partners, and suppliers in real time. To meet this need, Controlware also provides business intelligence solutions that analyze complex data and present actionable information in the form of executive dashboards, ad hoc reporting tools, and real-time databases with machine learning.
They also pride themselves on expertise in robotics, machine vision, statistical process control, data and content management. To ensure an optimized solution and overall customer satisfaction, they utilize a disciplined system design and development methodology. This enables them to provide exceptional value by using industry best practices, best-of-breed products and services, and robust project management in an open, collaborative, and transparent environment.
“FrameworX easily and cost effectively allowed us to handle many use cases and requirements that are hard and/or expensive to implement with other SCADA packages. It also contains multiple tools and interfaces for creating a modern flexible and open architecture system. The SCADA world is not just about working with PLC/Controllers anymore. FrameworX is built to integrate information from smart sensors, MES, ERP, and cloud. With its native .NET scripting, built-in drivers and data connectors, it allowed us to accomplish this without the need for third-party add-ins.”
— Richard Benamy, Controlware
